
Unpacking the impact of biosimilars
The pharmaceutical industry is at a crossroads of care where biosimilars can transform how we manage—and mitigate—rising specialty drug costs.
To understand key trends shaping the future of biosimilars, Evernorth Research Institute examined biosimilar utilization patterns and the influence of market forces in the latest Pharmacy in Focus report “The biosimilar breakthrough in adoption and affordability”.

Key insights
Key insights
Biosimilars are reshaping drug spend in the inflammatory conditions category, delivering real-world savings and bending the cost curve.
The growing biosimilar pipeline offers a critical lever to expand treatment options, lower costs, and improve outcomes for patients and communities.
Gaps in patient experiences and provider concerns can influence biosimilar adoption, highlighting the need to improve these areas.
Biosimilars are reshaping drug spend in the inflammatory conditions category, delivering real-world savings and bending the cost curve.
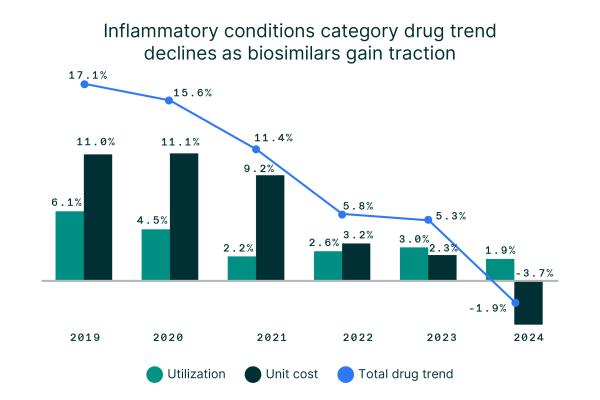
Data from Evernorth Research Institute shows a reversal in inflammatory conditions category drug trend—falling from 17.1% in 2019 to -1.9% in 2024. It marks the first sustained reversal in drug trend for inflammatory conditions in years, offering evidence that biosimilars are delivering on their promise of real-world savings.
1.9%
increase in drug utilization in 2024 despite unit costs dropping -3.7% from 2023
11%
unit cost in the inflammatory conditions category in 2019 compared to -3.7% in 2024
Biosimilars are bending the cost curve
January 2023: First Humira biosimilar launches
February 2023: Rapid steps were taken to ensure patients could use biosimilars
February 2024: First high-concentration, citrate- free, Humira biosimilar with interchangeable status approved
May 2024: First high-concentration, citrate-free interchangeable Humira biosimilar launches
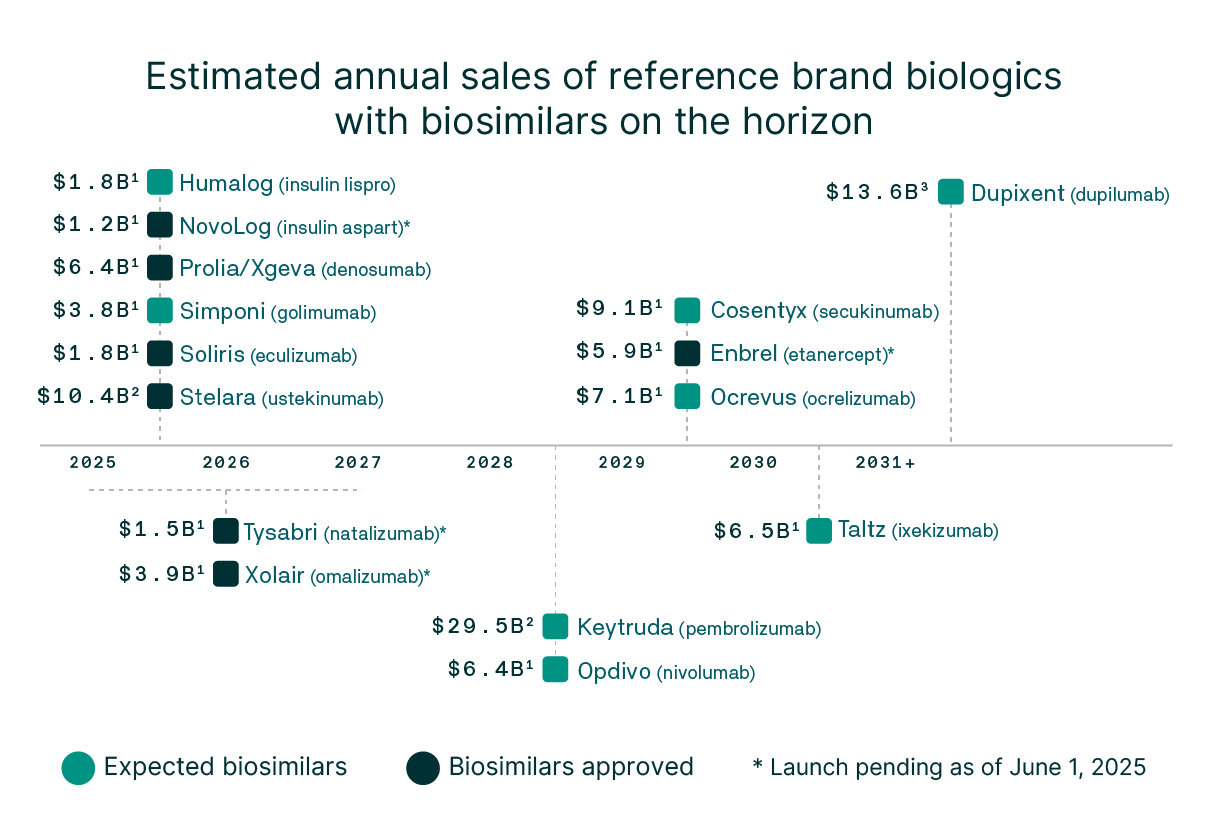
The growing biosimilar pipeline offers a critical lever to expand treatment options, lower costs, and improve outcomes for patients and communities
Biosimilar use and the price-lowering effect of market competition is not only increasing access to affordable therapies but is contributing to reducing costs—in an analysis of claims for Humira and related biosimilars, a commercial population experienced more than $200M in savings during a 15-month period.

Biosimilars on the horizon
Over the next 10 years, 118 biologics are expected to lose patent protection, representing a sizeable savings opportunity with biosimilars.
Gaps in adoption and provider barriers influence biosimilar adoption, highlighting the need to strengthen patient experiences and provider confidence
Evernorth Research Institute survey data shows that only 31% of patients living with a chronic condition are aware of biosimilars, compared to 64% of health care providers. This awareness gap underscores the crucial role providers play in introducing biosimilars and educating patients about this therapeutic option.

Inflammatory biosimilar utilization trend
Utilization is lower in younger generations who are demanding a system that listens, respects them and embraces whole person care.
Watch now
View the 30-minute on-demand webinar about the biosimilar breakthrough in adoption and affordability with Johanna Bellon and Meghan Pasicznyk.

Sources
- IPD Analytics. “Market & Financial Insights.” Last updated May 2025.
- Manalac T. “10 Best-Selling Drugs of 2024 Rake in Billions Amid Exclusivity Threats.” BioSpace. Accessed March 5, 2025.
Samorodnitsky D. “Sanofi Earnings Driven by Dupixent, New RSV Vaccine as Hunt for Deals Continues.” Biospace. Accessed January 30, 2025.
* Additional biosimilars for this product have been approved by the FDA but have not yet launched. Employers n=500. Providers n=526.
